Keynote Speakers
Dr. Yuan-Ting Zhang
Professor, City University of Hong Kong, ChinaSpeech Title: Health Engineering: Wearable “SUPER-MINDS” for the Precision Control of CVD and COVID
Abstract: The cardiovascular disease (CVD) and coronavirus disease (COVID) are the most current pressing health challenges globally today. This talk will attempt to address the grand challenges through the paradigm shift to Health Engineering based on a convergence approach to integrate technologies across multiple scales in the biological hierarchy from molecular, cell, organ to system. The presentation will focus on the development of unobtrusive wearable 'SUPER-MINDS' technologies and their integrations with biomarker sensing, medical imaging and machine learning for the early prediction of acute CVDs. Potential applications in the fast response and precise control of COVID-19 will also be discussed. Using the atherosclerotic plaque assessment as an example, this talk will illustrate that the health convergence approach should allow the practice of 8- P’s medicine that is predictive, preventive, precise, pervasive, personalized, participatory, preemptive, and patient-centralized.
Biography: Dr. Yuan-Ting Zhang is currently the Chair Professor of Biomedical Engineering at City University of Hong Kong and the Adjunct Chair Professor at Shandong University. He is a LRG member of Karolinska Institutet MWLC. He was the Sensing System Architect in Health Technology at Apple Inc., California, USA in 2015, and the founding Director of the Key Lab for Health Informatics of Chinese Academy of Sciences in 2007. Professor Zhang dedicated his service to the Chinese University of Hong Kong from 1994 to 2015 in the Department of Electronic Engineering, where he served as the first Head of the Division of Biomedical Engineering and the founding Director of the Joint Research Center for Biomedical Engineering.
Prof. Zhang serves as the Editor-in-Chief for IEEE Reviews in Biomedical Engineering, Chair of the Working Group for the development of IEEE 1708 Standard on Wearable Cuffless Blood Pressure Measuring Devices, Organizer of IEEE-MDBS series since 2002, Organizer of ISS-BHE series since 2007, and a member of IEEE Medal panel for Healthcare Technology Award since 2017. He was the Editor-in-Chief for IEEE Transactions on Information Technology in Biomedicine and the founding Editor-in-Chief of IEEE Journal of Biomedical and Health Informatics. He served as Vice Preside of IEEE EMBS, Technical Program Chair of EMBC’98 in Hong Kong, Conference Chair of EMBC’05 in Shanghai, International Committee Co-Chair of EMBC’07 in Lyon, Internationale Committee Chair of EMBC’ 11 in Boston, Internationale Committee Chair of EMBC’13 in Osaka, Technical Program Co-Chair of EMBC’17 in Jeju Island, and International Committee Co-Chair of EMBC’2020 in Montreal. He was the Chair of 2018 Gordon Research Conference on Advanced Health Informatics and Chair of 2016-2018 IEEE Award Committee in Biomedical Engineering. Prof. Zhang's research interests include cardiovascular health engineering, unobtrusive sensing and wearable devices, neural muscular modeling and pHealth technologies.
He was selected on the 2014, 2015, 2016, 2017, 2018 and 2019 lists of China’s Most Cited Researchers by Elsevier. He won a number of international awards including IEEE-EMBS best journal paper awards, IEEE-EMBS Outstanding Service Award, IEEE-SA 2014 Emerging Technology Award. Prof. Zhang is elected to be IAMBE Fellow, IEEE Fellow and AIMBE Fellow for his contributions to the development of wearable and m-Health technologies.
Dr. Eddie Y. K. Ng
Nanyang Technological University, SingaporeSpeech Title: Relation between Neck Skin Temperature Measurement and Carotid Artery Stenosis: In-Vitro Evaluation
Abstract: Carotid artery stenosis (CAS) is a form of atherosclerosis, where thrombus formation restricts the passage of blood through the carotid artery leading to irreversible damage in the brain tissue. The presence of stenosis in the carotid artery results in abnormal temperature maps on the external skin surface, which can be captured and quantified using non-contact/non-invasive infrared (IR) thermal imaging/thermography. In this study, a thermally charged in-vitro carotid artery flow loop, using 0% and 75% stenosis models, was designed to study the thermal effect on the external skin surface. The carotid artery flow was encapsulated with PDMS (polydimethylsiloxane) resembling neck tissue, of which the external surface temperature maps were studied using IR thermography. Using the mean temperature as a threshold value, the resultant thermal image was processed and normalized. Between the two stenosis models, disruption in the thermal features corresponding to the presence of stenosis was observed. The method described in this study paves the path to experimentally study the thermal effect of the presence of stenosis in the carotid artery.
Keywords: Atherosclerosis; Carotid artery stenosis; Conjugate bio-heat transfer; Infrared (IR) thermal imaging; Thermography; Thermal region of interest (TROI)
Technical Disclosure/Copyright:
- Ashish Saxena, E.Y.K. Ng, and Lim Soo Teik. “Neck Thermography for a Non-contact/Non-invasive Screening of Patients with Carotid Artery Stenosis”, NTUitive Pte. Ltd., Singapore (Ref. no.: 2019-046).
- Ashish Saxena, E.Y.K. Ng, Vignesh Raman, and Lim Soo Teik. “Thermography based superficial vein projection system to ease real-time vein puncturing”, NTUitive Pte. Ltd., Singapore (Ref. no.: 2018-150).
- Ashish Saxena, Vedabit Saha, E.Y.K. Ng, “Skin temperature maps as a measure of carotid artery stenosis”, Computers in Biology and Medicine (2020): 116, 103548 (13 pages), https://doi.org/10.1016/j.compbiomed.2019.103548.
- Ashish Saxena, E.Y.K. Ng, Vignesh Raman, Muhammad Syarifuddin Bin Mohamed Hamli, Mateusz Moderhak, Szymon Kolacz, and Jerzy Jankau. "Infrared (IR) Thermography-based Quantitative Parameters to Predict the Risk of Post-operative Cancerous Breast Resection Flap Necrosis." Infrared Physics & Technology (2019): 103, 103063 (12 pages), https://doi.org/10.1016/j.infrared.2019.103063.
- Ashish Saxena, E.Y.K. Ng, Soo Teik Lim, " Infrared (IR) thermography as a potential screening modality for carotid artery stenosis", Computers in Biology and Medicine (2019):113, 103419 (11 pages), https://doi.org/10.1016/j.compbiomed.2019.103419.
- Ashish Saxena, E.Y.K. Ng, Mrudang Mathur, Chirag Manchanda, and Nehal Amit Jajal, "Effect of carotid artery stenosis on neck skin tissue heat transfer." International Journal of Thermal Sciences 145 (2019): 106010, https://doi.org/10.1016/j.ijthermalsci.2019.106010.
- Ashish Saxena, E.Y.K. Ng, and Lim Soo Teik. "Imaging modalities to diagnose carotid artery stenosis: progress and prospect." BioMedical Engineering OnLine 18.1 (2019): 66, https://doi.org/10.1186/s12938-019-0685-7.
- Ashish Saxena, Vignesh Raman, and E.Y.K. Ng. “Study on methods to extract high contrast image in active dynamic thermography”, Quantitative InfraRed Thermography Journal (2019): 1-17, Taylor & Francis Online, https://doi.org/10.1080/17686733.2019.1586376.
- Ashish Saxena, E.Y.K. Ng, and Vignesh Raman, “Single Image Reconstruction in Active Dynamic Thermography: A Novel Approach”, Infrared Physics & Technology 93 (2018): 53-58, https://doi.org/10.1016/j.infrared.2018.07.020.
- Ashish Saxena, E.Y.K. Ng, and Vignesh Raman. "Thermographic Venous Blood Flow Characterization with External Cooling Stimulation." Infrared Physics & Technology 90 (2018): 8-19, https://doi.org/10.1016/j.infrared.2018.02.001.
- Tejas Canchi, Ashish Saxena, E.Y.K. Ng, and Sriram Narayanan. “Application of Fluid Structure Interaction Methods to estimate the mechanics of rupture in Abdominal Aortic Aneurysms”, BioNanoScience 8.4 (2018): 1035-1044, https://doi.org/10.1007/s12668-018-0554-z.
- Ashish Saxena and E.Y.K. Ng, “Steady and Pulsating Flow past a Heated Rectangular Cylinder(s) in a Channel”, Journal of Thermophysics and Heat Transfer 32.2 (2017): 401-413, https://doi.org/10.2514/1.T5265.
- A Saxena, EYK Ng, T Canchi, JL Lim, AS Beruvar, "A Method to Produce High Contrast Vein Visualization in Active Dynamic Thermography (ADT)", Journal of Medical Systems (2020) JOMS-D-20-00778, (in-press).
- A Saxena, EYK Ng, T Canchi, JJ Tee, LK Ng, “Relation between neck skin temperature measurement and carotid artery stenosis: in-vitro and in-vivo evaluation”, ASME Journal of Biomechanical Engineering. Vol. 142, 2020, pp. 114501-1, 8 pages, https://doi.org/10.1115/1.4048423.
- Ashish Saxena, EYK Ng, C Manchanda, Tejas Canchi, “Cardiac Thermal Pulse at the Neck Skin Surface as a Measure of Stenosis in the Carotid Artery”. Thermal Science and Engineering Progress Journal, 100603 (8 pages), 2020 Elsevier, https://doi.org/10.1016/j.tsep.2020.100603.
- Ashish Saxena, Ng, E. Y-K, Lim ST, “Active Dynamic Thermography to Predict the Presence of Stenosis in the Carotid Artery." Computers in Biology and Medicine, (2020), Vol. 120, pp. 103718 (9 pages), Elsevier, https://doi.org/10.1016/j.compbiomed.2020.103718.
Biography: Ng obtained Ph.D. at Cambridge Univ. and elected as a Fellow of The American Society of Mechanical Engineers; The Institution of Engineering and Technology [UK], and International Engineering & Technology Institute [HK]. He researches in numerical simulation in the biomedical engineering, thermal-fluids and health-related diagnosis fields. He is Editor-in-Chief for 2 ISI-journals which were captured by the JCR within 2-years of their inauguration. He has been recognized internationally for academic excellence. He received numerous best papers, service awards and has graduated 23 PhD and 26 Master students. He was awarded the SPRING-Singapore Merit Award for his work in thermal imagers to screen SARS fever and contributions to the Singapore Standardization Program. Twenty-one of his papers have been adopted as references in Singapore Standard (SS-582, Parts 1&2: 2020) and ISO/IEC 80601-2-59: 2017. He serves as a panel member for Singapore Biomedical and Health Standards Committee since 2011. Being a co-inventor of 3 US patents on software classifiers to identify the different stages of breast cancer development in iTBra-system, he was accoladed with equity in a listed company. His ongoing work on non-contact screening for carotid artery stenosis and superficial vein-finder has resulted in 3 TDs. He has notable citations in the field of infrared physics & technology.
Dr. Carlos Simmerling
Professor, Department of ChemistryAssociate Director, Laufer Center for Physical and Quantitative Biology, Stony Brook University, USA
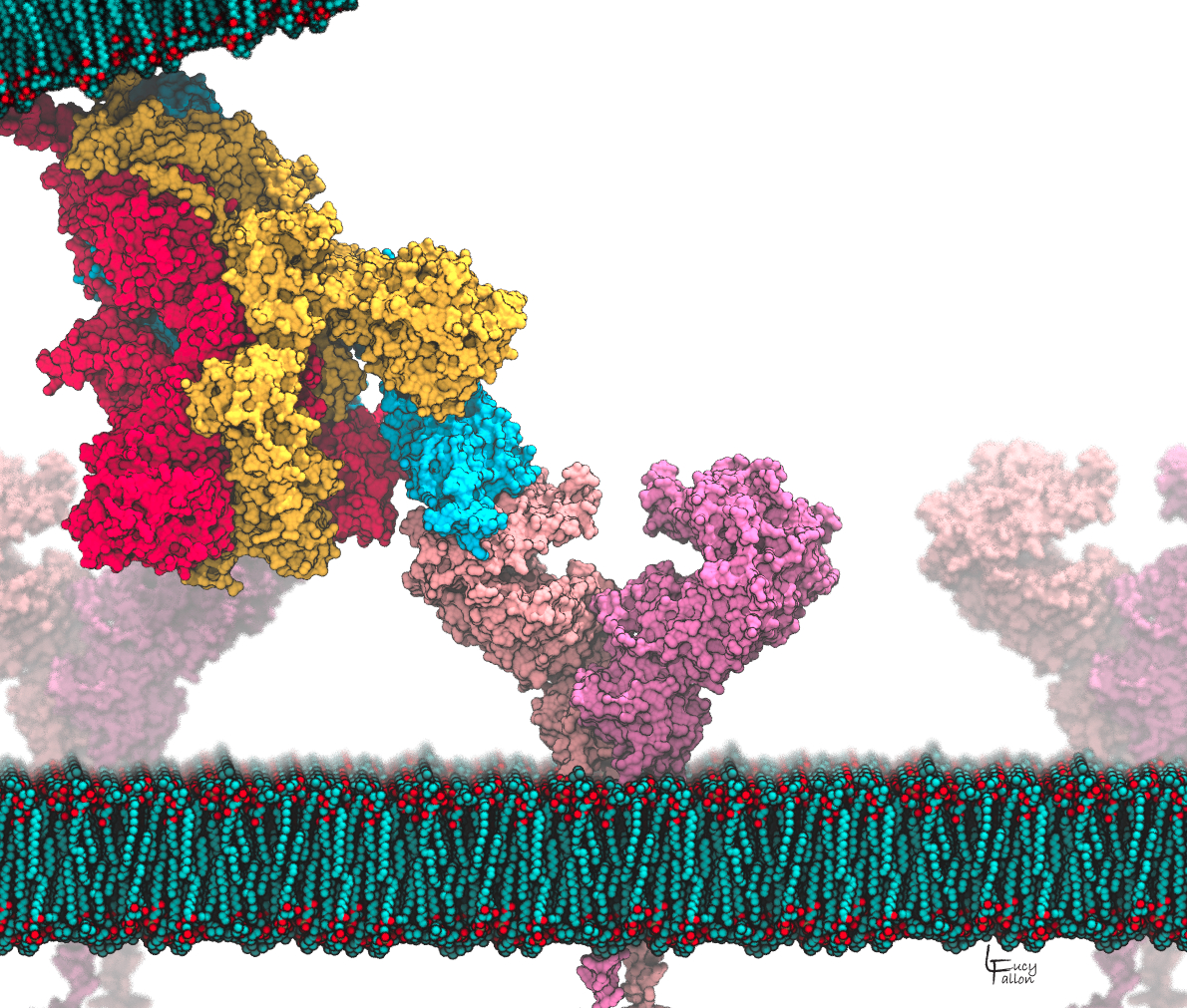
Speech Title: Using Computer Simulations to Explore Vulnerabilities in the SARS-Cov-2 Spike Glycoprotein
Abstract: Coronaviruses (CoVs) are so named due to the similarity of their appearance to a crown, with small protrusions of “spike” proteins covering their surface. The virus uses these spikes as molecular keys to unlock entry into and infect a host cell. Spike proteins are the key target of neutralizing antibodies, but development of immunity is slow. The spike has an extensive glycan shield that interferes with immune surveillance. Furthermore, antibodies tend to interact with the spike in the highly variable exposed regions, thus development of immunity to one CoV strain does not provide protection against another. We hypothesize that small molecule drugs could interact with the spike prior to immunity development, and block the conformational changes that are crucial to membrane fusion and infection. Modern drug discovery methods use structure to guide drug design, however these are hampered for COVID-19 because experimental structures are missing key regions for all coronavirus spikes, and none of the hypothesized membrane fusion steps have been directly observed. We are bridging this gap with computer models that can: complete the partial structures obtained from experiments, reveal the glycan shield absent in experimental models, provide mechanistic insight into viral evolution, and identify opportunities where drugs could block viral entry. Such drugs could have the potential to be broadly neutralizing of all CoVs, leading to effective treatments for COVID-19, as well as future pandemics caused by as-yet unknown CoVs.
Biography: Prof. Carlos Simmerling obtained his Bachelor’s degree (1991) and PhD (1994) in Chemistry at the University of Illinois at Chicago, performing early research on methods for computer modeling of biomolecules such as proteins. He went on to a post-doctoral fellowship in Pharmaceutical Chemistry at UCSF, where he became a lead developer of the Amber biomolecular simulation software that is used in thousands of research labs worldwide. In 1998 Prof. Simmerling joined the Chemistry department at Stony Brook University, where he is currently a Professor, and he became the Associate Director of SBU’s Laufer Center for Physical & Quantitative Biology. His research is funded by the USA National Institutes of Health, National Science Foundation, and Department of Energy. His work focuses on development of improved molecular simulation methods and models, and using these tools to study biomolecular recognition mechanisms. His articles on improving the physics underlying biomolecular modeling have been cited nearly 10,000 times. Prof. Simmerling is currently the Marsha Laufer Chair of Physical & Quantitative Biology at Stony Brook University and a Fellow of the American Chemical Society.
Dr. Mikael Kubista
ProfessorTATAA Biocenter AB, Gothenburg, Sweden
Speech Title: Two-Tailed PCR for Precision Diagnostics
Abstract: We present a highly specific, sensitive and cost-effective system to quantify miRNA, for typing of cell-free DNA in liquid biopsies and for direct blood genotyping based on novel chemistry called Two-tailed PCR. Two-tailed PCR takes advantage of target-specific primers composed of two hemiprobes complementary to two different parts of the target molecule connected by a hairpin structure. The introduction of a short hemiprobe that senses the variable sequences confers exceeding sequence specificity while maintaining the very high sensitivity of PCR. Highly similar targets can be distinguished with superior precision irrespectively of the position of the mismatched nucleotide. Further, the target molecule can be very short, making Two-tailed PCR the preferred method for microRNA profiling as well as analysis of rare sequence variants in cell-free (cf) DNA and formalin fixed paraffine embedded (FFPE) tissue. Two-tailed RT-qPCR has a dynamic range of 7 logs and a sensitivity sufficient to detect less than ten target miRNA molecules. Two-tailed PCR is readily multiplexed and for less challenging applications such as genotyping Two-tailed PCR can be applied directly on blood samples eliminating the need for extraction. This very smooth and friendly workflow is most suitable point-of-care testing at bedside, doctor’s office or in the field. Applications for cfDNA analysis are being developed by SimSen Diagnostics AB.
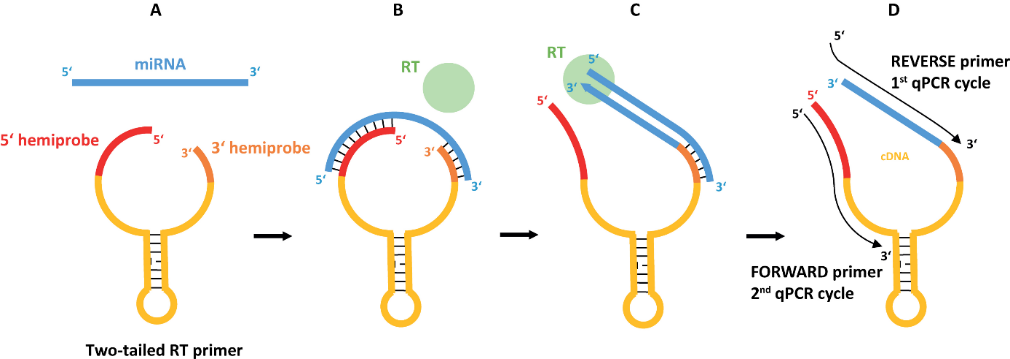
(1) Two-tailed RT-qPCR: a novel method for highly accurate miRNA quantification. P Androvic, L Valihrach, J Elling, R Sjoback, M Kubista. Nucleic acids research 45 (15), e144-e144
Biography: With a background as professor in Chemistry Kubista has become a serial entrepreneur in diagnostics. In 1996 he invented the light-up probes, which led to the foundation of LightUp Technologies AB as Europe´s first company focusing on qPCR-based diagnostics. In 2001 Kubista set up TATAA Biocenter as the first laboratory in Europe to obtain flexible ISO 17025 accreditation focuses on advanced services in molecular analyses to the pharmaceutical and biotech industries. in 2019 TATAA Biocenter was named “Best Nucleic Acid Analysis Service Provider – Europe” by Global Health & Pharma. Kubista introduced non-invasive prenatal testing (NIPT) in Sweden founding Life Genomics AB and direct blood genotyping co-founding LifeTest. Most recently TATAA invented the Two-tailed PCR for ultrasensitive nucleic acid detection, which has been licensed to Biovendor for microRNA analysis, and most recently SimSen Diagnostics was co-founded with the inventors of SimSen Sequencing to developed an ultrasensitive methods for molecular analyses of liquid biopsies. During the pandemic TATAA Biocenter was among the first in Europe testing for SARS-CoV-2 corona virus starting February 1, and is now contributing to the Swedish public testing effort as well as organizing testing of travellers through the platform www.coronapassport.se.
Dr. Catherine Green
Associate Professor, Head of the Clinical BioManufacturing FacilityNuffield Department of Medicine, University of Oxford, UK
Speech Title: The Development of the Oxford AstraZeneca COVID-19 Vaccine
Abstract: The Clinical Biomanufacturing Facility (CBF) at the University of Oxford (UK) produced the first clinical batches of the ChAdOx1 nCoV-19 vaccine against SARS-coV-2 in April 2020. This vaccine is now in phase III clinical trials across the globe, and in scaled up manufacture to produce millions of doses in the hope that it will provide effective protection against COVID-19. I will present the efforts that underpinned this highly rapid development of a vaccine against a provisionally unknown pathogen, the lessons that we have learnt, as well as the results generated from our early phase trials.
Biography: Catherine Green heads the Clinical Biomanufacturing Facility at the University of Oxford, she is an Associate Professor at the Wellcome Centre for Human Genetics, and a Senior Research Fellow at Exeter College Oxford. Her team at the CBF manufacture biologics at GMP for first in man and early phase clinical trials. Recently the CBF has been manufacturing simian Adenovirus vectored vaccines for a variety of pathogens with significant global disease burden, including Malaria, Zika, Rabies, Chikungunya. 2020 was a challenging year as we developed, manufactured, released and distributed into global clinical trials the ChAdOx1 nCoV-19 vaccine against COVID-19 which was recently acquired by AstraZeneca.





